The structure of a smell
10 May 2013
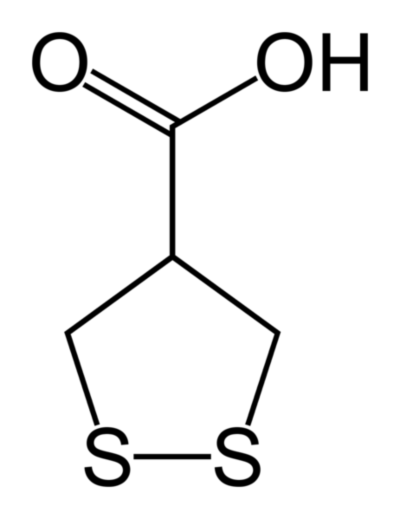
This is a post about the power of pictures and the smell of wee.
Chemicals, in the macroscopic, room-temperature-and-pressure world that we inhabit, are defined by what they do. Cyclopentane is a flammable liquid, a solvent, it smells a bit like petrol - and so on.
Structural formulae, to give them their proper appellation, are graphical representations of the arrangements of atoms within a molecule. Usually a few lines represent a carbon skeleton, and other atoms' elemental symbols are connected by lines representing covalent bonds. This is only one way of representing the information - it just happens to be pictorial and quick to sketch.
I’m struck by how powerful these little pictures are, how they exert a kind of magic attraction. To a non-chemist, neither a chemical’s name nor its molecular structure offer any useful clues to its properties. Draw a picture of that structure, though, and people nod appreciatively and feel they’ve “got it”. To paraphrase: “ah, now I know what that is”. Often, they’re none the wiser - but the feeling is palpable. “I know what the thing looks like, I can remember that, it ‘clicks’”.
In fact, the structural formula is usually redundant information, if you know the systematic name for a chemical. The one represented above is called 1,2-Dithiolane-4-carboxylic acid. Those 32 characters fully define the arrangement of the 14 atoms within the molecule. We know what it “looks like”. But do we know what it is?
Answer: a colourless solid, with a melting point of approximately 76°C. It’s a very seasonal compound. Its “common” name is asparagusic acid, and its smell (or rather, the smell of its metabolites) is the characteristic whiff of “asparagus wee”. My favourite vegetable at my favourite time of year, cryptically summed up by some little lines and a few letters!
Tags: chemistry, food, smell, information
< Previous post | Next post >Favourite posts
- On wiggly lines and being normal
- On infinite villages
- Running a race backwards
- Brainmaking
- Their tables were stored full, to glad the sight
- The structure of a smell
Recent posts
- Start your holidays with a meta-alarm
- PGN files from handwritten chess notation
- Souvenirs des villes européennes
- Pic'n'mix reinvented
- Super slow-mo Tetris
Blog archives
Posts from 2012, 2013, 2014, 2015, 2016, 2017, 2018, 2019, 2020, 2021, 2022, 2023, 2024.